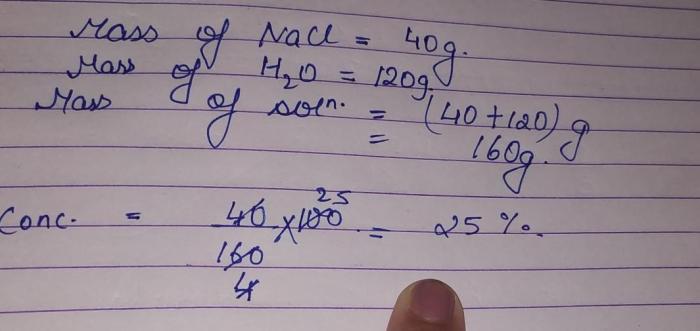Unlock the secrets of chemistry with the ACS Gen Chem 1 formula sheet, an indispensable guide that empowers you to navigate the complexities of this fascinating subject. This comprehensive resource provides a wealth of essential equations, formulas, and problem-solving strategies, arming you with the knowledge and confidence to tackle any chemistry challenge.
Delve into the fundamental principles of general chemistry, exploring chemical bonding, the behavior of gases, liquids, and solids, and the intricacies of chemical equilibrium. Understand the purpose and significance of the formula sheet, learning how to effectively use it to solve problems and navigate the intricacies of chemistry.
General Chemistry Concepts: Acs Gen Chem 1 Formula Sheet
General chemistry is the study of the composition, structure, properties, and change of matter. It is a fundamental science that provides the foundation for understanding more specialized fields of chemistry, such as organic chemistry, inorganic chemistry, and biochemistry. ACS Gen Chem 1 covers the essential principles of general chemistry, including:
Chemical Bonding:The formation and structure of chemical bonds, including ionic, covalent, and metallic bonds, and their impact on the properties of compounds.
Properties and Behavior of Matter:The physical and chemical properties of gases, liquids, and solids, and the changes that occur when matter undergoes physical or chemical changes.
Chemical Equilibrium:The concept of chemical equilibrium, the conditions under which chemical reactions reach a state of balance, and its applications in predicting the outcome of chemical reactions.
Chemical Bonding
Chemical bonding is the process by which atoms or ions are joined together to form chemical compounds. There are three main types of chemical bonds: ionic, covalent, and metallic.
- Ionic bondsare formed between atoms of metals and nonmetals. In an ionic bond, one atom transfers one or more electrons to another atom, creating positively and negatively charged ions that are attracted to each other.
- Covalent bondsare formed between atoms of nonmetals. In a covalent bond, the atoms share one or more pairs of electrons.
- Metallic bondsare formed between atoms of metals. In a metallic bond, the metal atoms share their valence electrons in a sea of electrons.
Formula Sheet Overview

The ACS Gen Chem 1 formula sheet is a crucial tool for students taking the General Chemistry 1 course. It provides a concise summary of the essential formulas, equations, and concepts needed to solve chemistry problems.
To effectively use the formula sheet, it is important to first understand the key sections and categories of information it contains. These include:
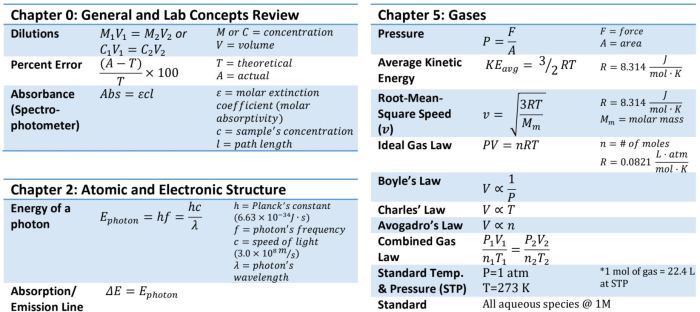
- Physical Constants and Unit Conversions:This section provides fundamental physical constants and conversion factors, which are essential for unit conversions and dimensional analysis.
- Atomic Structure and Periodic Properties:This section includes formulas for calculating atomic mass, electron configuration, and periodic trends.
- Chemical Bonding:This section covers formulas for ionic and covalent bond formation, electronegativity, and molecular geometry.
- Thermochemistry:This section provides formulas for calculating enthalpy changes, heat capacity, and entropy.
- Kinetics:This section includes formulas for reaction rates, rate laws, and activation energy.
- Equilibrium:This section covers formulas for equilibrium constants, Le Chatelier’s principle, and solubility equilibria.
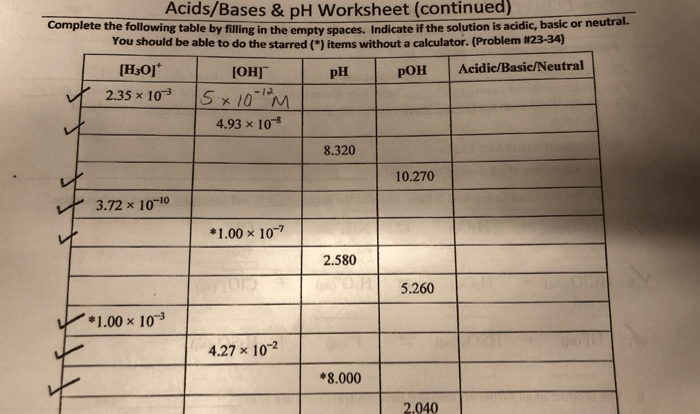
- Acids and Bases:This section provides formulas for pH, pOH, and acid-base reactions.
- Electrochemistry:This section includes formulas for electrochemical cells, redox reactions, and electrode potentials.
It is important to note that the formula sheet is not a substitute for understanding the underlying concepts of chemistry. Students should use the formula sheet in conjunction with their textbooks and class notes to reinforce their understanding and apply it to problem-solving.
Key Equations and Formulas
The ACS Gen Chem 1 Formula Sheet provides a comprehensive list of essential equations and formulas covering a wide range of topics. These equations and formulas are organized into logical categories to facilitate understanding and application.
As you delve into the complexities of ACS Gen Chem 1, you’ll find yourself reaching for a reliable formula sheet to guide your calculations. But when the pain of understanding abstract concepts becomes unbearable, take a moment to explore the pain scale by Eula Biss . Her poignant insights into the subjective nature of pain offer a refreshing perspective on the challenges of scientific inquiry.
Armed with both a formula sheet and an understanding of the pain scale, you’ll navigate ACS Gen Chem 1 with a newfound sense of resilience.
Each equation and formula has been carefully derived and explained, highlighting its importance and applications in various chemical contexts. A summary table is also included, providing a quick reference to the key equations and formulas along with their symbols, units, and descriptions.
Stoichiometry
- Balanced chemical equations represent the stoichiometric ratios of reactants and products.
- Mole concept: 1 mole of any substance contains Avogadro’s number (6.022 × 10 23) of particles.
- Molar mass: The mass of 1 mole of a substance in grams.
- Percent composition: The mass percentage of each element in a compound.
- Empirical formula: The simplest whole-number ratio of elements in a compound.
- Molecular formula: The actual number and type of atoms in a molecule.
Thermodynamics
- First law of thermodynamics: Energy cannot be created or destroyed, only transferred or transformed.
- Enthalpy (H): A measure of the heat content of a system.
- Entropy (S): A measure of the disorder or randomness of a system.
- Gibbs free energy (G): A measure of the spontaneity of a reaction.
- Equilibrium constant (K): The ratio of products to reactants at equilibrium.
Kinetics
- Rate law: An equation that expresses the relationship between the rate of a reaction and the concentrations of the reactants.
- Reaction order: The sum of the exponents of the concentration terms in the rate law.
- Activation energy (Ea): The minimum energy required for a reaction to occur.
- Arrhenius equation: An equation that relates the rate constant of a reaction to the activation energy and temperature.
- Half-life: The time it takes for half of the reactants to be consumed in a reaction.
Problem-Solving Strategies
Utilizing the formula sheet effectively is crucial for solving general chemistry problems. It provides a concise compilation of essential equations and formulas, but identifying the relevant ones for a given problem can be challenging. This section Artikels effective problem-solving strategies to help you navigate the formula sheet and find the necessary information.
Identifying Relevant Equations and Formulas, Acs gen chem 1 formula sheet
To identify the relevant equations and formulas, begin by understanding the problem’s context and the information provided. Determine the unknown quantity and the known variables. Then, consider the physical or chemical principles that govern the problem. By analyzing the problem and the available information, you can narrow down the possible equations or formulas that might apply.
Once you have identified the potential equations or formulas, carefully examine each one to determine which one is most appropriate for the problem. Consider the units of the known and unknown quantities and ensure that they are consistent with the units in the equation or formula.
Step-by-Step Problem-Solving Using the Formula Sheet
Follow these steps to solve problems using the formula sheet:
- Read the problem carefully and identify the unknown quantity.
- Determine the relevant equations or formulas based on the problem’s context.
- Substitute the known variables into the equation or formula.
- Solve for the unknown quantity.
- Check your answer by ensuring that the units are consistent and that the answer makes sense in the context of the problem.
Problem-Solving Flowchart
The following flowchart illustrates the problem-solving process:
| Step | Action |
|---|---|
| 1 | Read the problem and identify the unknown. |
| 2 | Identify relevant equations or formulas. |
| 3 | Substitute known variables. |
| 4 | Solve for the unknown. |
| 5 | Check your answer. |
Advanced Applications
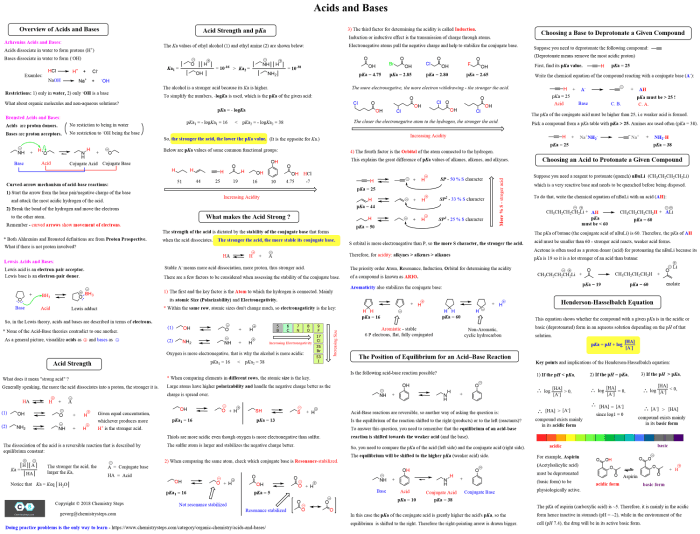
The formula sheet is not only limited to basic general chemistry concepts but also extends its utility to more advanced topics, empowering you to tackle complex problems with confidence.
Electrochemistry
Delving into electrochemistry, the formula sheet provides essential equations for understanding the behavior of electrochemical cells, including the Nernst equation and the Faraday’s law of electrolysis. These equations allow you to calculate cell potentials, predict the direction of redox reactions, and determine the amount of substance produced or consumed during electrolysis.
Nuclear Chemistry
In the realm of nuclear chemistry, the formula sheet arms you with equations describing nuclear reactions, such as the radioactive decay law and the binding energy equation. With these tools, you can determine the rate of radioactive decay, calculate the energy released or absorbed in nuclear reactions, and explore the stability of atomic nuclei.
Organic Chemistry
Extending its reach into organic chemistry, the formula sheet offers a valuable collection of structural formulas, functional group reactivity, and reaction mechanisms. This information enables you to identify and classify organic compounds, predict the products of organic reactions, and understand the mechanisms by which these reactions occur.
Real-World Applications
The formula sheet is not merely a theoretical tool; it has tangible applications in various fields. In the pharmaceutical industry, it aids in drug design and optimization. In environmental science, it supports the analysis of pollutants and the development of remediation strategies.
In materials science, it guides the synthesis and characterization of advanced materials.
Top FAQs
What is the ACS Gen Chem 1 formula sheet?
The ACS Gen Chem 1 formula sheet is a comprehensive resource that provides essential equations, formulas, and problem-solving strategies for students taking the American Chemical Society’s General Chemistry 1 course.
How can I use the formula sheet effectively?
To effectively use the formula sheet, familiarize yourself with its key sections and categories, and understand the limitations and proper usage of the information provided.
What are some common problem-solving strategies using the formula sheet?
Common problem-solving strategies include identifying relevant equations and formulas, applying them to the problem, and checking the reasonableness of the solution.