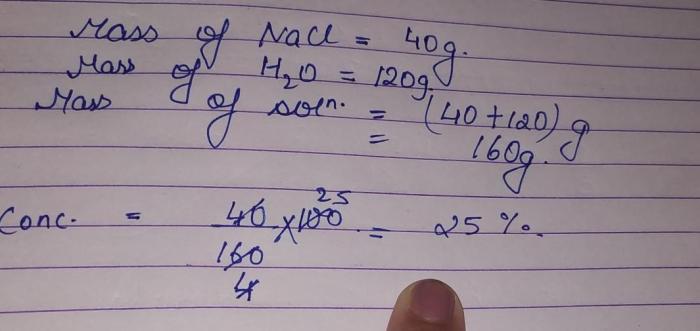Delving into the realm of chemistry, we present the percent composition empirical and molecular formulas worksheet answers, an invaluable resource for understanding the composition of compounds. This guide empowers you to unravel the mysteries of chemical formulas, providing a comprehensive overview of percent composition, empirical formulas, and molecular formulas.
Unveiling the intricacies of chemical compounds, this discourse delves into the fundamental concepts of percent composition, empirical formulas, and molecular formulas. With meticulous explanations and illustrative examples, we unravel the complexities of these concepts, empowering you with the knowledge to navigate the fascinating world of chemistry.
Percent Composition: Percent Composition Empirical And Molecular Formulas Worksheet Answers
Percent composition refers to the percentage by mass of each element in a compound. It is calculated by dividing the mass of each element by the total mass of the compound and multiplying by 100.
For example, if a compound has a mass of 100 g and contains 40 g of carbon, the percent composition of carbon in the compound would be (40 g / 100 g) x 100% = 40%.
Empirical Formulas
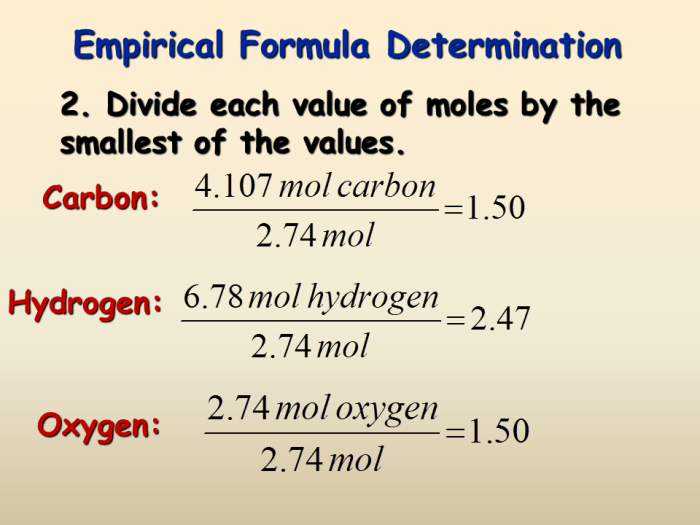
An empirical formula represents the simplest whole-number ratio of the elements in a compound. It is determined by finding the mass of each element in the compound and dividing each mass by the element’s atomic mass. The resulting numbers are then converted to the smallest whole-number ratio.
For example, if a compound is found to contain 40% carbon and 60% oxygen, the empirical formula would be CH 2O.
Molecular Formulas, Percent composition empirical and molecular formulas worksheet answers
A molecular formula represents the actual number of atoms of each element in a molecule of a compound. It is determined by multiplying the empirical formula by an integer that makes the molecular mass of the formula equal to the experimental molecular mass of the compound.
For example, if the empirical formula of a compound is CH 2O and the experimental molecular mass is 60 g/mol, the molecular formula would be C 2H 4O 2.
Worksheet Answers

| Question | Answer | Explanation |
|---|---|---|
| What is the percent composition of carbon in a compound with a mass of 100 g and contains 40 g of carbon? | 40% | (40 g / 100 g) x 100% = 40% |
| What is the empirical formula of a compound that contains 40% carbon and 60% oxygen? | CH2O | (40 g / 12 g/mol) = 3.33 mol C(60 g / 16 g/mol) = 3.75 mol ODivide by smallest number of moles:
Convert to whole numbers:
Round to nearest whole number:Empirical formula = CH2O |
| What is the molecular formula of a compound with an empirical formula of CH2O and an experimental molecular mass of 60 g/mol? | C2H 4O 2 | Molecular mass of CH2O = 30 g/mol(60 g/mol) / (30 g/mol) = 2Molecular formula = C 2H 4O 2 |
Key Questions Answered
What is percent composition?
Percent composition refers to the percentage by mass of each element present in a compound.
How do I calculate percent composition?
To calculate percent composition, divide the mass of each element by the total mass of the compound and multiply by 100.
What is an empirical formula?
An empirical formula represents the simplest whole-number ratio of elements in a compound.
How do I determine an empirical formula?
To determine an empirical formula, find the mass of each element in the compound and divide by its atomic mass. Then, divide each result by the smallest value obtained and simplify to whole numbers.
What is a molecular formula?
A molecular formula represents the actual number of atoms of each element in a molecule of a compound.
How do I determine a molecular formula?
To determine a molecular formula, divide the molar mass of the compound by the empirical formula mass. Then, multiply the subscripts in the empirical formula by this value to obtain the molecular formula.